CONSEP
A cluster-randomised controlled trial, implementing CONn Syndrome (primary aldosteronism) screening and Evaluation in Primary care (CONSEP).
CONn Syndrome (Primary aldosteronism), the commonest treatable cause of secondary hypertension, affects 5-14 per cent of general practice patients with hypertension. Missing a diagnosis of primary aldosteronism leads to adverse patient outcomes above and beyond hypertension. A simple blood test, aldosterone to renin ratio (ARR), is available to screen for this common and potentially curable condition, but is rarely done.
Background
The CONSEP project was awarded $2,299,203 by the Medical Research Future Fund in 2023.
Targeting an important gap in knowledge
Hypertension, the leading risk factor for death, affects about six million Australians. Around 10 per cent may have a potentially curable cause: primary aldosteronism (PA) or Conn syndrome. Hypertension in patients living with PA is poorly controlled by standard antihypertensive drugs and patients with PA are at two-four fold higher risk of heart disease and stroke. Early detection of PA using a simple blood test, the aldosterone to renin ratio (ARR), permits effective treatment or cure. However, the ARR test is significantly under-utilised, resulting in hundreds of thousands of Australians with PA missing out on accurate diagnosis and optimal care.
Testing health-related interventions
The pragmatic, parallel group, cluster-randomised controlled trial, CONSEP (CONn Syndrome Evaluation in Primary care), will test whether education and guidance-based requests (GBR) for general practitioners (GPs) can increase PA screening and diagnosis among patients living with hypertension. Informed by close collaboration with consumers, embracing the principles of implementation science and health economics, CONSEP will be conducted in 28 general practices in Victoria (VIC), Tasmania (TAS) and South Australia (SA).
Delivering relevant and implementable outcomes
CONSEP will establish the efficacy and cost-effectiveness of the intervention for increasing PA screening and diagnosis in primary care. If proven effective, the intervention can be rapidly translated into general practices around Australia to better control hypertension, improve lives and reduce health expenditure.
Partners
- Monash University
- University of Adelaide
- University of Tasmania
- Curtin University
- University of Queensland
Investigators
- Associate Professor Jun Yang
- Professor Gang Chen
- Professor Peter Fuller
- Dr StellaMay Gwini
- Dr Angela Melder
- Professor Mark Nelson
- Professor Christopher Reid
- Professor Grant Russell
- Professor Helen Skouteris
- Professor Nigel Stocks
- Professor Michael Stowasser
- Professor John Burgess
- Dr John Malios
- Associate Professor Ken Sikaris
- Professor David Torpy
- Mr David Wyatt
Project scope
CONSEP aims to develop and evaluate an evidence-based strategy to increase the screening and diagnosis of PA in people living with hypertension attending general practice.
Primary objectives
1. Compare the proportion of eligible patients screened for PA in the intervention vs control arm within the first 12 months post-intervention.
2. Compare the proportion of eligible patients diagnosed with PA in the intervention vs control arm within the first 24 months post-intervention (accounting for time taken to reach final diagnosis).
Secondary objectives
1. Compare the rates of BP control and amount of antihypertensive use in eligible patients in the intervention vs control arm at 12 and 24 months post-intervention.
2. Evaluate the process of implementing the intervention to increase the screening and diagnosis of PA
3. Evaluate the cost effectiveness of the intervention
Diagnostic pathway
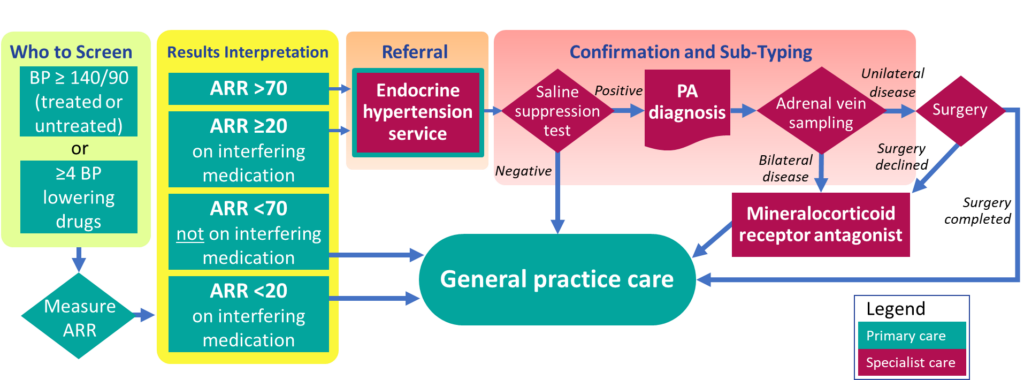
Who to screen
Treated or untreated patients with blood pressure ≥ 140/90 at 2 occasions, or patients currently treated with ≥ 4 blood pressure lowering drugs.
Where to refer
Patients who screen positive can be referred to;
Associate Professor Jun Yang
Endocrine Hypertension Service
Monash Health, Victoria
Referrals can be emailed to endocrinehypertensionclinic@monashhealth.org, or faxed to (03) 9594 3558.
Professor John Burgess
Endocrinology Outpatient Clinic
Royal Hobart Hospital, Tasmania
Referrals can be emailed to endocrinology.admin@ths.tas.gov.au
Professor David Torpy
Endocrine & Metabolic Service
Royal Adelaide Hospital, South Australia
Referrals can be faxed to (08) 8429-6074
The diagnostic pathway following screening of PA is shown in Fig 1. Diagnostic information should be feedback to the referring general practices and entered to Best Practice system.
Study design
The team will conduct a pragmatic, parallel-group, cluster randomised controlled trial in general practices in three Australian states. Assessments to address the objectives will be performed at before delivery of the intervention (T0), three months (T1), 12 months (T2) and 24 months (T3) after delivery of the intervention. These will be conducted for each site, each cluster and at different time points.
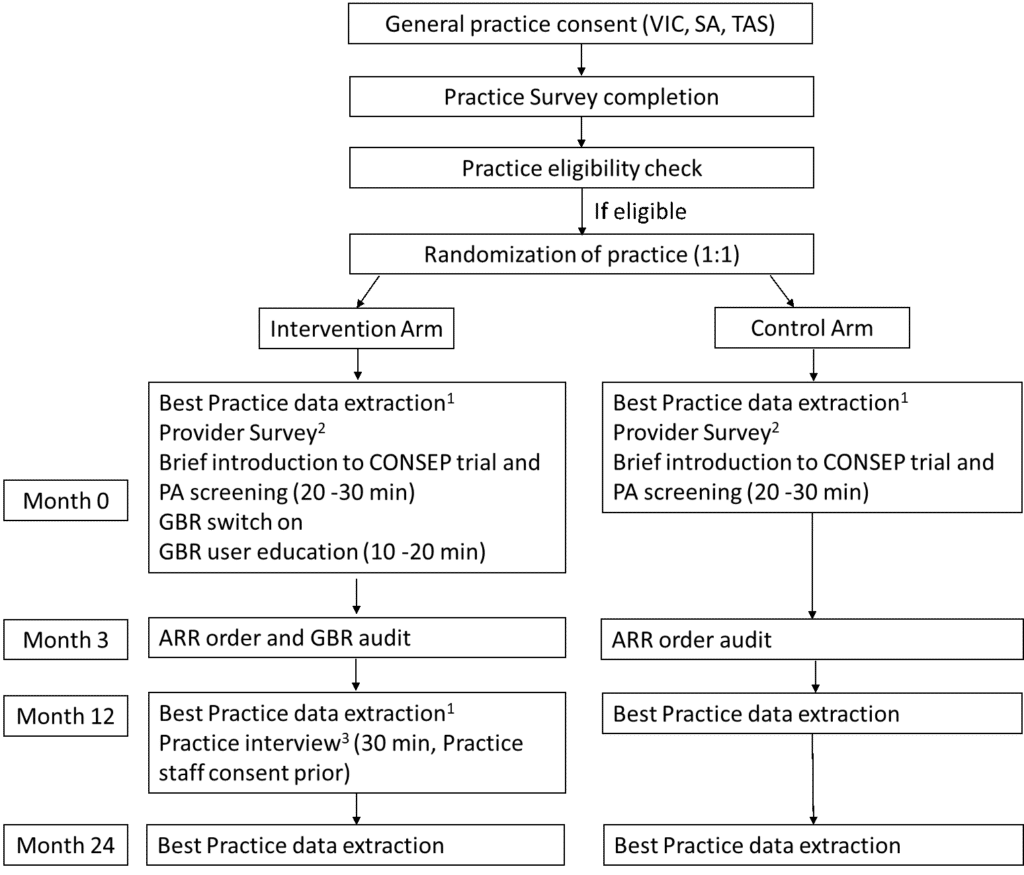
Figure 2: Summary of CONSEP trial design.
1. Best Practice data extraction needs to happen prior to the rest of the intervention or assessment at each time point. 2. Provider survey is optional. And the provider survey can be collected at any time before the intervention. 3. Practice interview is optional. Each practice interview could include a practice manager, at least two GPs and at most two other staff members that are heavily involved in the intervention. GBR: guidance based requesting..
Participants and eligibility criteria
General practices
Inclusion criteria
1. Use Best PracticeTM Clinical Management Software;
2. Be accredited;
3. Provide general primary care services;
4. Plan to be in operation for the next 2 years;
5. Use Sonic Pathology (Melbourne Pathology in VIC, Clinpath Pathology in SA, Hobart Pathology in TAS) as default pathology;
6. Both practices that share a database consented to participate and will be treated as one practice in randomization.
Exclusion criteria:
1. Practices recruited for our prior educational intervention
2. Practices which are previous users, current users or planning to use GBR in the next 2 years
3. Practices which share their database with more than one other practice.
Downloadable resources for patients
- Understanding primary aldosteronism
- Testing for primary aldosteronism
- Treating primary aldosteronism
Downloadable resources for General Practitioners
- Guidance Based Requesting User Guide
- Guidance Based Requesting Education
- PA and CONSEP Education Presentation 1
- PA and CONSEP Education Presentation 2

