Germline Stem Cell Biology
Germline stem cell
Overview
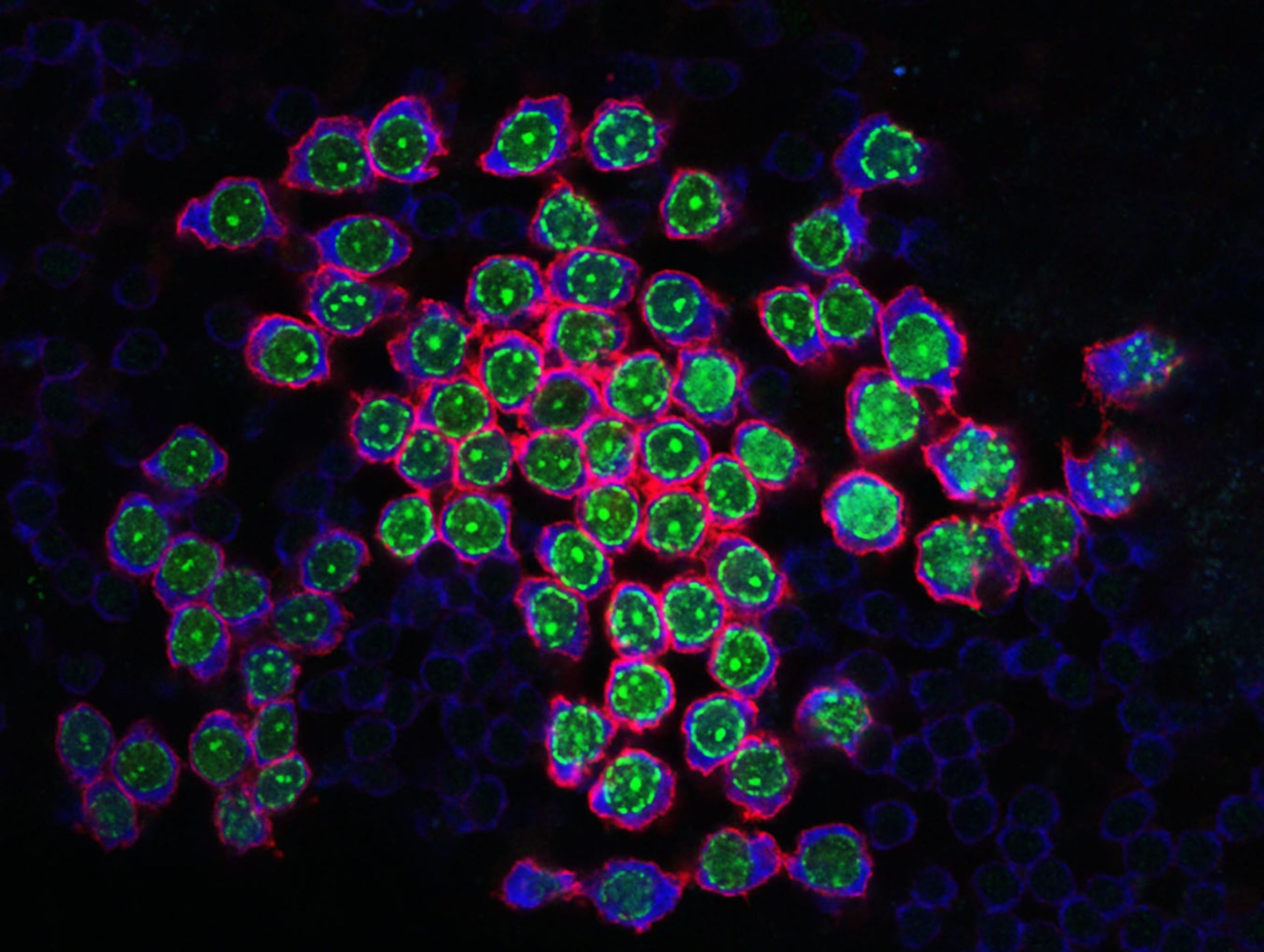
The maintenance of male fertility is dependent on spermatogonial stem cells (SSCs) that self-renew and generate differentiating germ cells for production of spermatozoa. After puberty, this process continuously produces mature sperm to maintain lifelong fertility. SSC function is dependent on specific growth factors produced within the testis microenvironment plus distinct cellular factors that regulate gene expression within SSCs and modulate responses to growth factor stimulation. However, despite the importance of SSCs for male fertility, the molecular mechanisms that regulate their function and maintenance remain incompletely understood.
Importantly, SSC function and male fertility can be compromised by multiple factors including ageing or exposure to genotoxic drugs. Increased paternal age is associated with disruption of SSC activity and declining sperm quality, with an elevated risk of genetic diseases in offspring. Infertility can also occur prematurely in men as a consequence of genotoxic therapies eg; chemotherapy, for treatment of cancer. However, cellular pathways mediating the regenerative response of SSCs following germline damage and loss of SSC function with age are poorly studied.
Our research focuses on defining genetic controls and cellular pathways regulating SSC function and male fertility. We employ a range of in vivo and in vitro experimental systems allowing dissection of mammalian SSC function. Our previous work has defined essential roles for the developmental transcription factors PLZF and SALL4 in maintenance of SSC activity and the central importance of mTORC1 signalling in SSC fate regulation. In addition, our studies have characterised cellular heterogeneity within the SSC and progenitor cell pool using single cell approaches and demonstrated the dynamic nature of spermatogonial states with important clinical implications.
Current research projects are focused on understanding cellular machinery modulating the response of SSCs to stimuli from the niche, the impacts of ageing on SSC function and molecular mechanisms supporting the regenerative capacity of SSCs.
Diseases we research
Research Group Head | Associate Professor Robin Hobbs
The maintenance of male fertility is dependent on spermatogonial stem cells that self-renew and generate differentiating germ cells for spermatozoa production. My research is defining the molecular mechanisms regulating spermatogonial stem cell function and male fertility.

News from the lab
Student opportunities

Publication highlights